How To Find The Formula Of A Compound
6.9: Calculating Molecular Formulas for Compounds
- Page ID
- 47495
Learning Objectives
- Sympathize the difference between empirical formulas and molecular formulas.
- Determine molecular formula from percentage composition and tooth mass of a chemical compound.
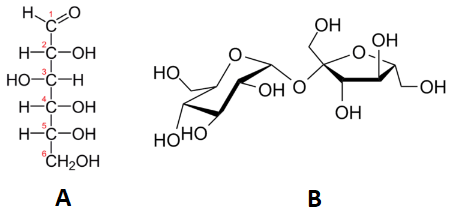
Below, nosotros meet ii carbohydrates: glucose and sucrose. Sucrose is virtually exactly twice the size of glucose, although their empirical formulas are very like. Some people can distinguish them on the basis of gustatory modality, just it's non a adept idea to become around tasting chemicals. The best mode to tell glucose and sucrose autonomously is to determine the molar masses—this approach allows you to hands tell which compound is which.
Molecular Formulas
Molecular formulas give the kind and number of atoms of each element nowadays in the molecular compound. In many cases, the molecular formula is the same equally the empirical formula. The chemical formula will always be some integer multiple (\(n\)) of the empirical formula (i.e. integer multiples of the subscripts of the empirical formula).
\[\text{ Molecular Formula} = due north (\text{Empirical formula})\]
therefore
\[ n = \dfrac{\text{Molecular Formula}}{\text{Empirical Formula}}\]
The integer multiple, north, tin also be obtained past dividing the molar mass, \(MM\), of the compound past the empirical formula mass, \(EFM\) (the molar mass represented past the empirical formula).
\[ north = \dfrac{MM ( molar mass)}{EFM (empirical formula molar mass)}\]
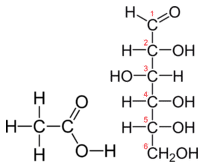
Tabular array \(\PageIndex{1}\) shows the comparison between the empirical and molecular formula of methane, acerb acid, and glucose, and the different values of north. The molecular formula of marsh gas is \(\ce{CH_4}\) and considering it contains only 1 carbon atom, that is likewise its empirical formula. Sometimes, still, the molecular formula is a uncomplicated whole number multiple of the empirical formula. Acetic acid is an organic acid that is the master component of vinegar. Its molecular formula is \(\ce{C_2H_4O_2}\). Glucose is a uncomplicated carbohydrate that cells utilise as a primary source of energy. Its molecular formula is \(\ce{C_6H_{12}O_6}\). The structures of both molecules are shown in Figure \(\PageIndex{ii}\). They are very different compounds, nonetheless both have the same empirical formula of \(\ce{CH_2O}\).
| Name of Compound | Molecular Formula | Empirical Formula | n |
|---|---|---|---|
| Methane | \(\ce{CH_4}\) | \(\ce{CH_4}\) | 1 |
| Acetic acid | \(\ce{C_2H_4O_2}\) | \(\ce{CH_2O}\) | 2 |
| Glucose | \(\ce{C_6H_{12}O_6}\) | \(\ce{CH_2O}\) | 6 |
Empirical formulas can be adamant from the percent composition of a chemical compound as discussed in section 6.8. In order to determine its molecular formula, information technology is necessary to know the molar mass of the compound. Chemists utilise an musical instrument called a mass spectrometer to determine the tooth mass of compounds. In society to go from the empirical formula to the molecular formula, follow these steps:
- Calculate the empirical formula molar mass (EFM).
- Divide the molar mass of the compound by the empirical formula molar mass. The result should be a whole number or very close to a whole number.
- Multiply all the subscripts in the empirical formula by the whole number plant in step ii. The result is the molecular formula.
Instance \(\PageIndex{ane}\)
The empirical formula of a compound of boron and hydrogen is \(\ce{BH_3}\). Its molar mass is \(27.7 \: \text{m/mol}\). Decide the molecular formula of the compound.
Solution
| Steps for Trouble Solving | Determine the molecular formula of \(\ce{BH_3}\). |
|---|---|
| Identify the "given" information and what the problem is asking you to "detect." | Given: Empirical formula \(= \ce{BH_3}\) Molar mass \(= 27.seven \: \text{g/mol}\) Find: Molecular formula \(= ?\) |
| Calculate the empirical formula mass (EFM). | \[\text{Empirical formula tooth mass (EFM)} = 13.84 \: \text{g/mol} \nonumber\] |
| Divide the molar mass of the compound past the empirical formula mass. The result should exist a whole number or very shut to a whole number. | \[\frac{\text{molar mass}}{\text{EFM}} = \frac{27.7 g/mol}{13.84 g/mol} = 2 \nonumber \] |
| Multiply all the subscripts in the empirical formula by the whole number found in step 2. The upshot is the molecular formula. | \[\ce{BH_3} \times 2 = \ce{B_2H_6} \nonumber \] |
| Write the molecular formula. | The molecular formula of the compound is \(\ce{B_2H_6}\). |
| Think virtually your issue. | The molar mass of the molecular formula matches the molar mass of the chemical compound. |
Exercise \(\PageIndex{ane}\)
Vitamin C (ascorbic acid) contains 40.92 % C, 4.58 % H, and 54.50 % O, past mass. The experimentally determined molecular mass is 176 amu. What are the empirical and chemical formulas for ascorbic acrid?
- Answer Empirical Formula
- C3H4O3
- Answer Molecular Formula
- CviHviiiOsix
Summary
- A procedure is described that allows the adding of the verbal molecular formula for a compound.
Contributions & Attributions
This page was synthetic from content via the following correspondent(s) and edited (topically or extensively) by the LibreTexts development squad to meet platform way, presentation, and quality:
-
Marisa Alviar-Agnew (Sacramento City College)
-
Henry Agnew (UC Davis)
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map:_Introductory_Chemistry_%28Tro%29/06:_Chemical_Composition/6.09:_Calculating_Molecular_Formulas_for_Compounds
Posted by: ryanlesse1976.blogspot.com


0 Response to "How To Find The Formula Of A Compound"
Post a Comment